What is the maximum Sr2+ concentration possible in a solution that has a 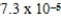 M sulfide-ion concentration without precipitating strontium sulfate? For SrSO4,Ksp = 2.5 × 10-7.
M sulfide-ion concentration without precipitating strontium sulfate? For SrSO4,Ksp = 2.5 × 10-7.
A) 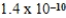 M
M
B) 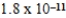 M
M
C)  M
M
D) 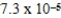 M
M
E) 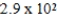 M
M
Correct Answer:
Verified
Q74: If 500 mL of 1.3 × 10-6
Q75: Solid KCN is added to a solution
Q76: What is the minimum concentration of Pb2+
Q77: What will happen if 0.1 mol of
Q78: Sodium chloride is added slowly to a
Q80: Which of the following solutions should be
Q81: What is the value of the dissociation
Q82: For which of the following salts would
Q83: The figure below represents the results of
Q84: Suppose hydrogen sulfide is added to a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents