Given the two equilibria below,
Ag(NH3) 2+(aq)  Ag+(aq) + 2NH3(aq) ; Kd = 5.9 × 10-8AgI(s)
Ag+(aq) + 2NH3(aq) ; Kd = 5.9 × 10-8AgI(s) 
Ag+(aq) + I−(aq) ; Ksp = 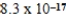
What is Kc for the following equilibrium?AgI(s) + 2NH3(aq) 
Ag(NH3) 2+(aq) + I-(aq)
A) 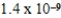
B) 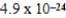
C) 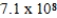
D) 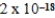
E) 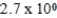
Correct Answer:
Verified
Q104: What will happen if 50.0 mL of
Q105: A _ is an ion formed from
Q106: Given the following equilibrium constants,
Zn(IO3)2 Ksp =
Q107: Determine the molar solubility of AgCl in
Q108: What is the molar solubility of nickel(II)sulfide
Q110: The following reaction represents a step in
Q111: In the sulfide scheme for qualitative analysis,the
Q112: _ involves the determination of the identity
Q113: A(n)_ is a Lewis base that bonds
Q114: In the qualitative analysis scheme for metal
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents