Given the following,determine ΔG°f at 298 K for SnO.
Sn(s) + SnO2(s) → 2SnO(s) ; ΔG° = 12.0 kJ at 298K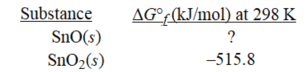
A) -251.9 kJ/mol
B) -503.8 kJ/mol
C) 527.8 kJ/mol
D) 263.9 kJ/mol
E) 1055.6 kJ/mol
Correct Answer:
Verified
Q39: For which of the following processes would
Q40: Which of the following reactions has the
Q41: Based on the following data,what is the
Q42: Consider the following reaction:
CaO(s)+ CO2(g)→ CaCO3(s); ΔG°
Q43: For a reaction system that is at
Q45: For which of the following substances is
Q46: Determine ΔG° for the following reaction:
CH4(g)+ 2O2(g)
Q47: What is ΔS° at 298 K for
Q48: Which of the following is correct for
Q49: -Given the following,determine ΔG° at 298 K
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents