The standard free energy of formation of nitric oxide,NO,at 1000.K (roughly the temperature in an automobile engine during ignition) is 77.7 kJ/mol.Calculate the equilibrium constant for the reaction 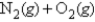

2NO(g)
At 1000.K.(R = 8.31 J/(K ∙ mol) )
A) 0.95
B) 7.6 × 10-9
C) 1.6 × 105
D) -15
E) 8.7 × 10-5
Correct Answer:
Verified
Q69: A 0.0835 M solution of a particular
Q70: For the reaction 4Ag(s)+ O2(g)→ 2Ag2O(s),ΔH° =
Q71: What is ΔG° at 298 K for
Q72: For a reaction that has an equilibrium
Q73: A certain reaction is found to be
Q75: For the reaction CaCO3(s)→ CaO(s)+ O2(g)at 1
Q76: The reaction CaO(s)+ SO3(g)→ CaSO4(s)is nonspontaneous at
Q77: Water gas,a commercial fuel,is made by the
Q78: Consider the following reaction:
2AgCl(s)→ 2Ag(s)+ Cl2(g); ΔH°
Q79: What is ΔG° at 500.0 K for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents