Water gas,a commercial fuel,is made by the reaction of hot coke carbon with steam: 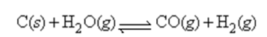
When equilibrium is established at 832°C,the concentrations of CO,H2,and H2O are 4.00 × 10-2,4.00 × 10-2,and 1.00 × 10-2 mol/L,respectively.Calculate the value of ΔG° for this reaction at 832°C.
A) 55.0 kJ
B) 12.7 kJ
C) 16.8 kJ
D) -12.7 kJ
E) none of these
Correct Answer:
Verified
Q72: For a reaction that has an equilibrium
Q73: A certain reaction is found to be
Q74: The standard free energy of formation of
Q75: For the reaction CaCO3(s)→ CaO(s)+ O2(g)at 1
Q76: The reaction CaO(s)+ SO3(g)→ CaSO4(s)is nonspontaneous at
Q78: Consider the following reaction:
2AgCl(s)→ 2Ag(s)+ Cl2(g); ΔH°
Q79: What is ΔG° at 500.0 K for
Q80: Consider the following reaction:
2C(s)+ 2H2(g)→ C2H4(g); ΔH°
Q81: Sublimation is an example of a process
Q82: Which of the following statements is true
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents