Balance the following half-reaction occurring in acidic solution.
NO3-(aq) → 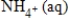
A) NO3-(aq) + 10H+(aq) + 8e− → 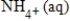 + 3H2O(l)
+ 3H2O(l)
B) NO3-(aq) + 3H2O(l) + 10e− → 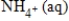 + 10H+(aq)
+ 10H+(aq)
C) NO3-(aq) + 10H+(aq) → 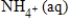 + 3H2O(l) + 10e−
+ 3H2O(l) + 10e−
D) NO3-(aq) + 8e− → 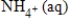 + 8H+(aq) + 3H2O(l)
+ 8H+(aq) + 3H2O(l)
E) NO3-(aq) + 10H+(aq) → 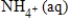 + 3H2O(l)
+ 3H2O(l)
Correct Answer:
Verified
Q12: When the following oxidation-reduction reaction in acidic
Q13: The anode in a voltaic cell and
Q14: Which of the following statements is true
Q15: The electrochemical reaction which powers a lead-acid
Q16: According to the following cell notation,which species
Q18: A lead storage battery involves the following
Q19: Which of the following statements is true
Q20: When the following oxidation-reduction reaction in acidic
Q21: What is the cell reaction for the
Q22: For a galvanic cell using Fe |
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents