Multiple Choice
The cell potential of an electrochemical cell with the cell reaction
2Al(s) + 3Zn2+(aq) → 3Zn(s) + 2Al3+(aq)
Is 1.607 V.What is the maximum electrical work obtainable from this cell when 2.0 mol of Al is consumed?
A) 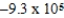 J
J
B) 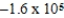 J
J
C) 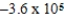 J
J
D) 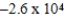 J
J
E) 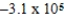 J
J
Correct Answer:
Verified
Related Questions
Q26: Given: Q27: The cell potential of an electrochemical cell Q28: In the following electrochemical cell,what is the Q29: A fuel cell designed to react grain Q30: What is the cell reaction for the Q32: What is the balanced spontaneous reaction and
Mn2+(aq)+ 2e- ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents