Consider the following cell reaction:
2Cr(s) + 6H+(aq) → 2Cr3+(aq) + 3H2(g) ; E°cell = 0.74V
Under standard-state conditions,what is E° for the following half-reaction?
Cr3+(aq) + 3e− → Cr(s)
A) -0.74 V
B) 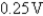
C) -0.37 V
D) 0.37 V
E) 0.74 V
Correct Answer:
Verified
Q48: Given: Q49: Given: Q50: Which of the following statements is true Q51: Given: Q52: Given: Q54: Given: Q55: Consider the following cell reaction: Q56: Consider the following reduction potentials: Q57: Which of the following is true for Q58: Given: Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents
W3+(aq)+ 3e- ![]()
Pb2+(aq)+ 2e- ![]()
Zn2+(aq)+ 2e- ![]()
Zn2+(aq)+ 2e- ![]()
Pb2+(aq)+ 2e- ![]()
2Hg2+(aq)+ H2(g)→ 2H+(aq)+
Cd2+(aq)+ 2e-
Li+(aq)+ e- ![]()

