If the cell is initially at standard-state conditions,which of the following statements is true? 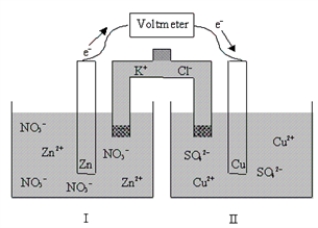 Zn2+(aq) + 2e-
Zn2+(aq) + 2e-  Zn(s) ; E° = -0.76 V
Zn(s) ; E° = -0.76 V
Cu2+(aq) + 2e-  Cu(s) ; E° = 0.34 V
Cu(s) ; E° = 0.34 V
A) Initially Kc = 2 × 10-37,and it decreases with time.
B) Initially Kc = 2 × 1037,and it does not change with time.
C) Initially Kc = 2 × 1037,and it decreases with time.
D) Initially Kc = 2 × 1037,and it increases with time.
E) Initially Kc = 2 × 10-37,and it increases with time.
Correct Answer:
Verified
Q75: Which of the following statements is true
Q76: For a reaction in a voltaic cell,both
Q77: If E°cell for a certain reaction is
Q78: If the value of E°cell is 2.10
Q79: What is E of the following cell
Q81: A voltaic cell is made by placing
Q82: Given: Q83: What is one of the major products Q84: For the cell Cu(s)|Cu2+||Ag+|Ag(s),the standard cell potential Q85: Given:![]()
Zn2+(aq)+ 2e- ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents