A sodium nucleus, 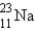 ,is bombarded with alpha particles to form a radioactive nuclide.This product nuclide can decay by several routes.Which of the following sets of products does not represent a potential route of decay of the product nuclide?
,is bombarded with alpha particles to form a radioactive nuclide.This product nuclide can decay by several routes.Which of the following sets of products does not represent a potential route of decay of the product nuclide?
A) 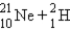
B) 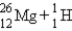
C) 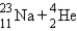
D) 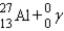
E) 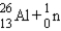
Correct Answer:
Verified
Q1: Which particle has the same mass as
Q2: When the radioactive nuclide 110In undergoes positron
Q4: When 103Pd undergoes electron capture,what is the
Q5: Which of the following statements about 248Bk
Q6: Which of the following nuclides will produce
Q7: When the radioactive nuclide 62Zn undergoes electron
Q8: The gamma ray emission from the decay
Q9: Which particle does the nuclide symbol
Q10: The radioactive nuclide,22Na,undergoes decay by emitting a
Q11: Which of the following nuclides is most
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents