How much energy is released when 2.00 metric tons of 2H2 gas undergoes nuclear fusion? (1 metric ton = 1000 kg,c = 3.00 × 108 m/s,1 amu = 1.66054 × 10-27 kg)
2H + 2H → 3He + 1n
Particle
Mass (amu) 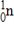
1) 008665 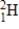
2) 01400 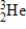
3) 01603
A) 6.77×10-18J
B) 1.48×1017J
C) 1.07×1017J
D) 5.39×1010J
E) 2.95×1017J
Correct Answer:
Verified
Q64: Which of the following nuclides has the
Q65: Which of the following statements is incorrect?
A)Isotope
Q66: A radioactive isotope undergoes decay by emission
Q67: What quantity of energy is released per
Q68: A 4.50-mg sample of a newly discovered
Q70: One of the first nuclear bombs based
Q71: The half-life of phosphorus-33 is 25 days.How
Q72: The isotope 210Pb has a half-life of
Q73: What assumption must be true for radiocarbon
Q74: Strontium-90 is produced in nuclear explosions.It can
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents