The major industrial source of hydrogen gas is the reaction of methane and water at high temperatures (800-1000°C) and high pressures (10-15 atm) with nickel as a catalyst. 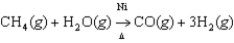 If 150.0 g of CH4 and 150.0 g of H2O are reacted at 915°C and 11.0 atm,how much hydrogen should be available for industrial use?
If 150.0 g of CH4 and 150.0 g of H2O are reacted at 915°C and 11.0 atm,how much hydrogen should be available for industrial use?
A) 171 L
B) 1330 L
C) 249 L
D) 25.0 L
E) 222 L
Correct Answer:
Verified
Q64: Which of the following reactions does not
Q65: A sample of pure deuterium oxide,D2O,is mixed
Q66: Which of the following is not an
Q67: Highly pure silicon for the manufacture of
Q68: Which of the following statements concerning oxides
Q70: Catenation is
A)the ability of a compound to
Q71: Dinitrogen gas is prepared commercially
A)from the decomposition
Q72: Which property of carbon distinguishes it from
Q73: Which of the following metals tends to
Q74: Nitric acid can be obtained directly by
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents