Near a pH of 5.5,L-lysine (R = −(CH2) 4NH2) the major species in aqueous solution is the diprotonated zwitterion.Given the following acid dissociation constants,what is the correct structure of the zwitterion?
Functional Group
Ka
Carboxylic acid
1) 7 x10-2
Amine
8) 5 x10-10
Side chain amine
1) 51 x10-11
A) 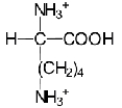
B) 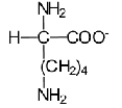
C) 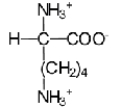
D) 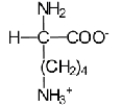
E) 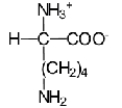
Correct Answer:
Verified
Q27: The nucleic acid sequence that is complementary
Q28: Which of the following amino acids has
Q29: Polymers of amino-acid units are called
A)metabolites.
B)nucleic acids.
C)lipids.
D)carbohydrates.
E)proteins.
Q30: What is the functional group corresponding to
Q31: When L-lysine (R = −(CH2)4NH2)is reacted with
Q33: The sequence of amino acids held together
Q34: Which of the following amino acids has
Q35: Which of the following structures is the
Q36: What are the building blocks of nucleic
Q37: The overall shape of a protein is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents