System 1 is 3.6 moles of a monatomic ideal gas held at a constant volume as it undergoes a temperature increase.System 2 is 3.6 moles of a diatomic ideal gas held at constant volume as it also undergoes a temperature increase.The thermal energy absorbed by System 1 is 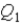
,and the thermal energy absorbed by System 2 is 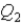
As both systems undergo a temperature increase of 74.6 K.What is the ratio 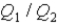
?
A) 1.00
B) 0.60
C) 1.67
D) The volumes of the systems need to be known before the ratio can be found.
E) 2.50
Correct Answer:
Verified
Q1: A 2.0-mol ideal gas system is maintained
Q4: On a PV diagram,an _ process is
Q7: In an isovolumetric process by an ideal
Q12: During an isobaric process which one of
Q16: A quantity of monatomic ideal gas expands
Q22: A heat engine operating between a pair
Q24: An electrical power plant manages to send
Q26: When gasoline is burned,it gives off 47,000
Q38: According to the second law of thermodynamics,which
Q50: In which system is heat usually transferred
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents