For the n = 5 shell,what are the lowest values possible for 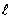
And 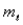
Respectively?
A) -5,-5
B) −4,-4
C) 0,-4
D) 0,0
Correct Answer:
Verified
Q22: How many possible substates are available in
Q23: The quantum mechanical model of the hydrogen
Q24: What is the next energy level that
Q25: The ground state electronic configuration for magnesium
Q26: If the principal quantum number for hydrogen
Q29: For n = 6,what are the maximum
Q30: The quantum mechanical model of the hydrogen
Q31: The quantum mechanical model of the hydrogen
Q47: How many electrons are in chlorine's (atomic
Q51: The ground state configuration for an element
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents