One in 5600 water molecules contains a deuterium atom.If all the deuterium could be extracted from 2 m3 of water and then reacted,how much energy could be obtained? (Each D-D fusion liberates 3.65 MeV of energy, 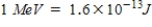
, 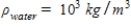
,one mole of water has a mass of 18 g,and 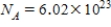
)
A) 7.0E+12 J
B) 2.2E+25 J
C) 5.8E+11 J
D) 3.5E+12 J
E) 1.1E+15 J
Correct Answer:
Verified
Q18: Calculate the energy released in the following
Q27: What is meant by a particle being
Q31: The weak force that acts between an
Q34: Which of the following particles is (are)considered
Q35: Assuming the Lawson criterion for the deuterium-tritium
Q36: The formation of a star requires the
Q41: If a negative muon decays to form
Q44: Calculate the range of the force that
Q46: "MeV/c2" is a unit for
A)energy.
B)mass.
C)momentum.
D)nuclear force.
Q50: The virtual exchange of photons can produce
A)a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents