A sample of pure carbon weighing 1.48 g was burned in an excess of air.The mass of carbon dioxide,the sole product,was 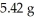
.In a second experiment,11.62 g of carbon dioxide was obtained.What mass of carbon was burned in the second experiment?
A) 42.6 g
B) 3.17 g
C) 3.54 g
D) 0.866 g
E) 11.6 g
Correct Answer:
Verified
Q55: A cubic centimeter of lead weighs 11.35
Q56: How many moles are represented by 2.5
Q57: With mass spectral data the ratio of
Q58: The total number of neutrons in an
Q59: How many atoms of rubidium-85 are in
Q61: The average atomic mass of B is
Q62: The following ratios of masses were obtained
Q63: Copper occurs in an isotopic mixture of
Q64: An anion has 45 neutrons and 36
Q65: The three naturally occurring isotopes of magnesium
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents