A cation has 13 neutrons and 10 electrons.If it has a charge of +1,what is its correct symbol?
A) 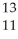
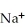
B) 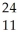
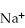
C) 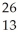
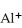
D) 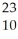
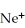
E) 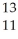
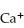
Correct Answer:
Verified
Q72: Write the symbol for the most common
Q73: What is the proper Q74: Write the appropriate symbol for the species Q75: The total numbers of neutrons,protons,and electrons in Q76: The total numbers of neutrons,protons,and electrons in Q78: Silver possesses two stable isotopes: 107Ag (106.90 Q79: How many protons,neutrons,and electrons are in Q80: Rubidium possesses two stable forms and has Q81: What is the mass of a sample Q82: Iodine is a member of the family![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents