To produce ferrocene one requires 2.33 g of C5H5 (65.0 g/mol) for every 1.00 g of Fe used (55.9 g/mol) .What is the value of x in the formula of ferrocene: 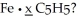
A) 1
B) 2
C) 3
D) 4
E) 5
Correct Answer:
Verified
Q71: From the following percent by mass compositions,determine
Q72: The element M forms the chloride MCl4
Q73: 4.72 g of a compound of carbon,hydrogen,and
Q74: A strip of electrolytically purified copper weighing
Q75: A 4.05 g sample of a compound
Q77: An organic compound contains C,H,and O.The percentages
Q78: The molecular formula for a sugar molecule
Q79: The complete combustion of 0.285 g of
Q80: A compound has the following percentage composition,by
Q81: The oxidation state of oxygen in oxygen
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents