The chemical reaction occurring during the discharge of a lead storage battery can be represented by the equation: 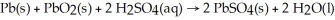
What mass of lead sulfate would result from the complete reaction of 41.4 g of lead?
A) 57.6 g
B) 60.5 g
C) 105 g
D) 115 g
E) 121 g
Correct Answer:
Verified
Q62: If an aqueous solution containing 46 g
Q63: What is the molarity of a sucrose
Q64: Write the complete balanced equation for the
Q65: What is the molarity of formaldehyde in
Q66: What is the sum of the coefficients
Q68: What is the molarity of methanol,
Q69: Our task is to measure the volume
Q70: What is the sum of the coefficients
Q71: What is the sum of the coefficients
Q72: What is the sum of the coefficients
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents