Identify the coefficient of NO(g) when the following reaction is balanced,and calculate the volume of nitrogen monoxide gas produced when 8.00 g of ammonia is reacted with 12.0 g of oxygen at 25 °C.The density of nitrogen monoxide at 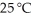
Is 1.23 g L-1.
(unbalanced) NH3(g) + O2(g) → NO(g) + H2O(l)
A) coefficient 4,volume 7.32 L
B) coefficient 1,volume 11.1 L
C) coefficient 2,volume 11.5 L
D) coefficient 3,volume 17.3 L
Correct Answer:
Verified
Q160: How many grams of NaOH (MW =
Q161: Calculate the concentration (M)of sodium ions in
Q162: How many millilitres of 0.260 mol L-1
Q163: What is the molar concentration of sodium
Q164: How many grams of CaCl2 are formed
Q166: When 31.2 mL of 0.500 mol L-1
Q167: How many grams of H2 gas can
Q168: How many millilitres of 0.200 mol L-1
Q169: How many moles of Co2+ (aq)are present
Q170: If 100.mL of 0.400 mol L-1 Na2SO4(aq)is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents