53.5 g of an ideal gas of molecular weight = 30.5 g/mol is confined at a pressure of 133 mmHg.The density of the gas is 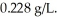 Compute the temperature of the gas in degrees Celsius.
Compute the temperature of the gas in degrees Celsius.
A) 261 °C
B) 12 °C
C) -57 °C
D) 285 °C
E) -12 °C
Correct Answer:
Verified
Q23: What volume would be occupied by 4.8
Q24: Three volumes of O2 and one volume
Q25: In the reaction:
2 Al(s)+ 6 HCl(aq)→ 2
Q26: Subtracting the vapor pressure of water from
Q27: If 0.50 mole H2(g)and 1.0 mole He(g)are
Q29: A balloon with volume 750 cm3 is
Q30: Of the following gases,the one with the
Q31: A gaseous mixture consists of 50.0% O2,25.0%
Q32: What volume of acetylene gas,C2H2,would be required
Q33: In the reaction ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents