Chloroform (CHCl3) became popular as an anesthetic after Queen Victoria delivered her eighth child while anesthetized by chloroform in 1853.What is the density in grams per liter of chloroform at 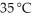
And 850 Torr?
A) 4.21 g/L
B) 0.190 g/L
C) 2.14 g/L
D) 4.00 × 103 g/L
E) 5.28 g/L
Correct Answer:
Verified
Q63: Butane (C4H10)is used as a fuel where
Q64: What pressure must be applied to N2(g)to
Q65: Calculate the volume of H2(g)expressed at STP,required
Q66: A 500.0 mL sample of O2(g)is at
Q67: A 10.0 L container of unknown gas
Q69: Chloroform became popular as an anesthetic after
Q70: What is the density of carbon dioxide
Q71: Halothane (CHBrClCF3)is one of the modern anesthetics
Q72: A sample of gas weighs 0.250 g
Q73: A 5.00 L container of unknown gas
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents