The mole fraction of nitrous oxide in dry air near sea level is 1.818 × 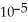
,where the molar mass of nitrous oxide is 44.013.The concentration of nitrous oxide in the atmosphere is ________ ppm.
A) 2.0 × 1012
B) 5.0 × 10-5
C) 500
D) 18.18
E) 5.0 × 10-13
Correct Answer:
Verified
Q98: If 250.L of nitrogen reacts with 582
Q99: What volume would be occupied by a
Q100: When 8.21 L of C3H8(g)is completely burned
Q101: A container filled with gas is connected
Q102: If the pressure in a gas container
Q104: What is the pressure in a gas
Q105: What is the average speed (actually the
Q106: Which of the following gases has the
Q107: Which of the following gases has the
Q108: Three identical flasks contain three different gases
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents