Determine the enthalpy change in the following equation: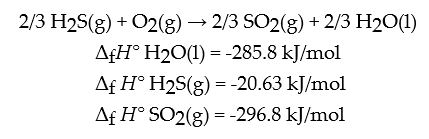
A) -562 kJ/mol
B) -375 kJ/mol
C) 375 kJ /mol
D) -402 kJ/mol
E) 402 kJ/mol
Correct Answer:
Verified
Q79: Many people believe that coffee 
Q80: 246 g of hot coffee at 86.0
Q81: Determine the enthalpy change in the following
Q82: Determine the enthalpy change in the following
Q83: Given that ΔfH°[CO(g)] = -110.5 kJ/mol and
Q85: Combine the reactions:
P4(s)+ 6 Cl2(g)→ 4 PCl3(g)ΔrH°298
Q86: Determine △rH° for the decomposition of hydrogen
Q87: Given the following thermochemical equations: Q88: H2S(g)+ 3/2 O2(g)→ H2O (l)+ SO2(g)ΔrH° = Q89: Some "beetles" defend themselves by spraying hot![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents