Electromagnetic radiation with a wavelength of 525 nm appears as green light to the human eye.The energy of one photon of this light is ________ J.
A) 1.04 × 10-31
B) 3.79 × 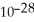
C) 3.79 × 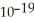
D) 1.04 × 10-22
E) 2.64 × 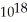
Correct Answer:
Verified
Q107: Which ion does not have a noble
Q108: How many valence shell electrons does an
Q109: The element that corresponds to the electron
Q110: What are the possible values of n
Q111: The complete electron configuration of nickel,element 28,is:
A)
Q113: How many subshells are there in the
Q114: How many orbitals are there in the
Q115: The condensed electron configuration of sulfur,element 16,is:
A)[Ne]3s2
B)[Ar]3s2
C)[Ne]3s23p4
D)[He]2s42p8
E)[He]3s2
Q116: For a hydrogen atom,which electronic transition would
Q117: Electromagnetic radiation with a wavelength of 531
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents