Which statement is INCORRECT about molecular orbital theory?
A) The number of molecular orbitals produced is equal to the number of atomic orbitals combined.
B) Each pair of sigma molecular orbitals is a bonding orbital and an antibonding orbital.
C) The antibonding orbital is at a lower energy than the bonding orbital.
D) The bond order (BO) is 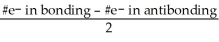 .
.
E) Hund's rule says that each orbital of identical energy has one electron before pairs are formed.
Correct Answer:
Verified
Q54: According to MO theory,assuming that the molecular
Q55: If the HCOO- ion is described using
Q56: The extra stability that a molecule gains
Q57: According to MO theory,which is the INCORRECT
Q58: Which of the following statements concerning molecular
Q60: The concept of an anti-bonding orbital is
Q61: What would be the hybridization of the
Q62: Which is the correct molecular orbital diagram
Q63: If the wave functions describing the 2s
Q64: How many σ bonds and how many
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents