Multiple Choice
What is ΔrG°?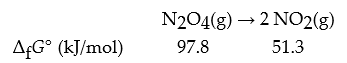
A) 149.1 kJ
B) -4.8 kJ
C) -46.5 kJ
D) 4.8 kJ
E) 46.5 kJ
Correct Answer:
Verified
Related Questions
Q85: For CO(g)+ H2(g)→ H2CO(g),ΔrH° = -5.36 kJ/mol,and
Q86: For CdO(s)+ SO3(g)→ CdSO4(s),ΔrH° = -279.4 kJ/mol,and
Q87: For the reaction N2O3(g)→ NO(g)+ NO2(g)ΔrG° =
Q88: For Cl2O(g)+ 3/2 O2(g)→ 2 ClO2 ,ΔrH°
Q89: Consider the reaction: N2O4(g)? 2 NO2(g)
Q91: For the reaction SO2(g)+ Cl2(g)→ SO2Cl2(g)Keq =
Q92: Consider the reaction: Q93: Calculate ΔrG° for the reaction Cu(s)+ H2O(g)→ Q94: Consider the following reaction: Q95: What is ΔrG°? Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()

