The solubilities of ammonium bromide,NH4Br,in water at 0 °C,20 °C,and 80 °C are as follows: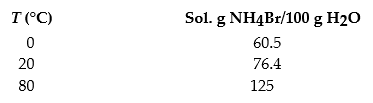
Which of the following fractional crystallization schemes would produce the highest percent yield for the recrystallization of ammonium bromide?
A) A solution containing 50.5 g NH4Br in 100.0 g H2O at 20 °C is cooled to 0 °C.
B) A solution containing 115 g NH4Br in 200 g H2O at 80 °C is cooled to 0 °C.
C) A solution containing 120 g NH4Br in 100 g H2O at 80 °C is cooled to 20 °C.
D) A solution containing 100 g NH4Br in 100.0 g H2O at 80 °C is cooled to 20 °C.
E) A solution containing 95 g NH4Br in 175 g H2O at 80 °C is cooled to 0 °C.
Correct Answer:
Verified
Q35: Which of the following compounds has the
Q36: Which compound is likely to be the
Q37: An azeotropic mixture is a:
A)mixture of two
Q38: Which of the following organic substances is
Q39: Henry's Law constants for aqueous solutions at
Q41: Which of the following aqueous solutions has
Q42: Choose the INCORRECT statement.
A)Colloidal particles are large
Q43: Which of the following is an example
Q44: Which of the following material-colloid type combinations
Q45: Colligative properties are similar in that they
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents