A solution is prepared by dissolving 7.00 g of glycerin ( C3H8O3) in 201 g of ethanol 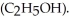 The freezing point of the solution is ________ °C.The freezing point of pure ethanol is
The freezing point of the solution is ________ °C.The freezing point of pure ethanol is 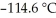 .The molal freezing point depression constant (
.The molal freezing point depression constant ( 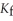 ) for ethanol is
) for ethanol is 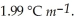 The molar masses of glycerin and of ethanol are 92.1 g mol-1 and 46.1 g mol-1,respectively.
The molar masses of glycerin and of ethanol are 92.1 g mol-1 and 46.1 g mol-1,respectively.
A) -121.3
B) 0.752
C) -107.9
D) -113.8
E) -115.4
Correct Answer:
Verified
Q120: What volume of an aqueous solution of
Q121: What is the expected freezing point of
Q122: Which of the following solutions will have
Q123: An aqueous solution has a normal boiling
Q124: What is the freezing point of a
Q126: Calculate the freezing point of a solution
Q127: A KCl solution is prepared by dissolving
Q128: A solution is prepared by adding 30.00
Q129: At 20 °C,a 0.376 mol L-1 aqueous
Q130: At a given temperature the vapour pressures
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents