Consider the following equilibrium reaction: PCl3(g) + Cl2(g) ⇌ PCl5(g) Kc = 96.2 The equilibrium constant for the decomposition of PCl5(g) to PCl3(g) and Cl2(g) is ________.
A) -Kc
B) 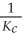
C) 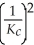
D) 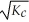
E) 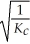
Correct Answer:
Verified
Q43: Equilibrium constant K is constant except when
Q44: For the production of NO2,Kc = [NO2]2/[NO]2[O2].At
Q45: Consider the following reaction at a certain
Q46: For the following reaction
O2(g)⇌ 2 O(g)
What conditions
Q47: For CO2(g)+ H2(g)⇌ CO(g)+ H2O(g),Kc = [CO][H2]/[CO2][H2],if
Q49: 0.75 mol of N2 and 1.20 mol
Q50: For the reaction;N2(g)+ 3 H2(g)⇌ 2 NH3(g),the
Q51: Consider the reaction:
2 SO2(g)+ O2(g)⇌ 2 SO3(g),ΔrH°
Q52: 2.5 moles H2O and 100 g of
Q53: For the reaction: CH4(g)+ 2 H2O(g)⇌ CO2(g)+
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents