The acid-dissociation constant of hydrocyanic acid (HCN) at 25.0 °C is 4.9 × 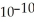
.What is the pH of an aqueous solution of 0.080 mol L-1 sodium cyanide (NaCN) ?
A) 11.11
B) 2.89
C) 1.3 × 10-3
D) 7.8 × 10-12
E) 3.9 × 10-11
Correct Answer:
Verified
Q118: Calculate the pH of a 0.800 mol
Q119: Calculate the hydronium ion concentration in an
Q120: A solution with a hydrogen ion concentration
Q121: Which one of the following salts,when dissolved
Q122: The base-dissociation constant of ethylamine (
Q124: Calculate the pH of a 0.080 mol
Q125: What is the pH of a 0.40
Q126: Which one of the following salts,when dissolved
Q127: What is the pH of a 0.30
Q128: Calculate the pH of a 1.60 mol
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents