The following half-reactions are used in the zinc-air battery.
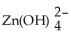 (aq) + 2 e- → Zn(s) + 4 OH-(aq) E° = -1.199 V
(aq) + 2 e- → Zn(s) + 4 OH-(aq) E° = -1.199 V
O2(g) + 2 H2O(l) + 4 e- → 4 OH-(aq) E° = +0.401 V
How much charge is transferred per gram Zn(s) in this voltaic cell?
A) 5.90 × 103 C
B) 2.95 × 103 C
C) 1.47 × 103 C
D) 3.39 × 10-4 C
E) 1.69 × 10-4 C
Correct Answer:
Verified
Q45: Determine E°cell for the reaction: Pb(s)+ Cu2+(aq)→
Q46: Determine E°cell for the reaction: Cl2(g)+ 2
Q47: Determine E°cell for the reaction:
Pb(s)+ Zn2+(aq)→
Q48: Determine E°cell for the reaction: I2(s)+ Cu(s)→
Q49: Write the net redox reaction that occurs
Q51: For the reaction: Mg(s)+ 2 AgNO3(aq)→ 2
Q52: Write the net redox reaction that occurs
Q53: How many coulombs would be needed to
Q54: Determine E°cell for the reaction: 2 Al3+(aq)+
Q55: One mole of electrons has a charge
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents