A voltaic cell is constructed with two 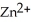 -Zn electrodes,where the half-reaction is
-Zn electrodes,where the half-reaction is 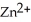 (aq) +
(aq) + 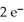 → Zn(s) E° = -0.763 V
→ Zn(s) E° = -0.763 V
The concentrations of zinc ion in the two compartments are 5.50 mol L-1 and 1.11 × 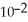
Mol L-1,respectively.The cell emf is ________ V.
A) -1.54 × 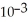
B) -378
C) 0.0798
D) 0.160
E) -0.761
Correct Answer:
Verified
Q103: Given that E°red = -1.66 V for
Q104: What is the balanced chemical equation for
Q105: What is the shorthand notation that represents
Q106: A voltaic cell is constructed with two
Q107: The gas OF2 can be produced from
Q109: What is the reduction half-reaction for the
Q110: Consider the following standard reduction potentials:
Ni2+(aq)+ 2
Q111: Consider the galvanic cell, Q112: Based on the following information, Q113: A solution of AuCl3(aq)is electrolyzed by passing![]()
F2(g)+ 2 e-
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents