Data for the reaction A + B → C are given below.Find the rate constant for this system. 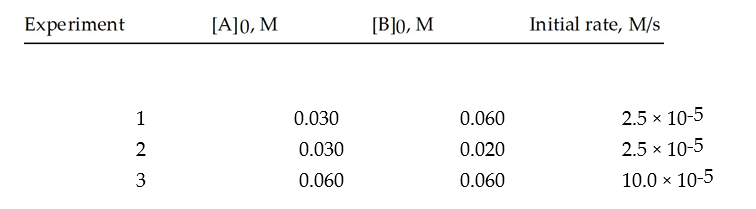
A) 2.8 × 10-2 M-1s-1
B) 2.8 × 10-2 Ms-1
C) 2.8 × 10-2 M2s-1
D) 1.7 × 10-3 M-1s-1
E) 1.7 × 10-3 Ms-1
Correct Answer:
Verified
Q19: In the reaction C4H9Cl(aq)+ H2O(l)→ C4H9OH(aq)+ HCl(aq)the
Q20: In the Arrhenius equation,ln k = -Ea/RT
Q21: Which of the following is FALSE for
Q22: In a second order reaction:
I.the sum of
Q23: For a reaction Rate = k[A][B]2,what factor
Q25: The reaction A + B → C
Q26: Which of the following has no effect
Q27: Activation energy is:
A)energy at the bottom of
Q28: Choose the INCORRECT statement.
A)A reaction intermediate is
Q29: Which of the following statements is correct?
A)A
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents