Which is a plausible mechanism for the following reaction if the rate law is 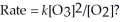
2 O3 → 3 O2
A) 2 O3 → O2 + O4 (slow)
O4 → 2 O2 (fast)
B) 2 O3 → O6 (slow)
O6 → O4 + O2 (fast)
O4 → 2 O2 (slow)
C) 2 O3 → O4- + O2+ (slow)
O4- → O2 + O2- (fast)
O2- + O2+ → 2 O2 (slow)
D) O3 ⇌ O2 + O∙ (fast)
O∙ + O3 → 2 O2 (slow)
E) O3 ⇌ O2+ + O- (fast)
O- + O3 → O2 + O2- (slow)
O2- + O2+ → 2 O2 (fast)
Correct Answer:
Verified
Q46: For the reaction: 2 N2O5(g)→ 4 NO2(g)+
Q47: For the reaction: 2 N2O5(g)→ 4 NO2(g)+
Q48: Why is rate = k[HgCl2] 2[C2O42-] not
Q49: Which of the following lowers the activation
Q50: What is the rate constant at 305
Q52: What is the main difference between a
Q53: Which statement is INCORRECT?
A)Activation energy is always
Q54: A factor that decreases the activation energy
Q55: What is the rate law for the
Q56: For the reaction: 2 N2O5(g)→ 4 NO2(g)+
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents