The first order reaction A → products has 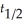 = 150 s.What percent of the sample remains unreacted after 300 s?
= 150 s.What percent of the sample remains unreacted after 300 s?
A) 25%
B) 50%
C) 12.5%
D) 0.0%
E) 100%
Correct Answer:
Verified
Q58: According to the collision theory in gaseous
Q59: What is the rate law for the
Q60: In the Arrhenius equation,ln k = -Ea/RT
Q61: If the half-life of a reaction depends
Q62: A catalyst:
I.lowers activation energy
II.provides an alternate reaction
Q64: Which of the following situations involves a
Q65: Data for the reaction A + B
Q66: The reaction 2 H2 + NO →
Q67: For the reaction: C2H4Br2 + 3 KI
Q68: The rate constant for a first-order reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents