Write the equation for the production of potassium metal from potassium chloride.
A) KCl(s) + Na(s) 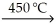 NaCl(s) + K(s)
NaCl(s) + K(s)
B) KCl(s) + Na(s) 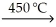 KNaCl(s)
KNaCl(s)
C) KCl(l) + Na(l) 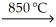 NaCl(l) + K(g)
NaCl(l) + K(g)
D) KCl(l) + Na(g) 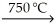 NaCl(s) + K(s)
NaCl(s) + K(s)
E) There is no reaction.
Correct Answer:
Verified
Q78: Choose the INCORRECT statement about carbon.
A)In addition
Q79: Choose the INCORRECT statement about silicon.
A)Silicon forms
Q80: Choose the INCORRECT statement.
A)The strong C-C and
Q81: What is the pH of a solution
Q82: Quartz and mica are examples of:
A)silanols
B)silanes
C)silicones
D)silicates
E)isomers
Q84: Write the equation for the electrolysis of
Q85: What mass of potassium superoxide could be
Q86: Write the chemical equation for the reaction
Q87: A sample of water has hardness expressed
Q88: Calculate the number of grams of aluminum
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents