The production of lead is a two step process:
2 PbS(s) + 3 O2(g) 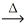 2 PbO(s) + 2 SO2(g)
2 PbO(s) + 2 SO2(g)
2 PbO(s) + C(s) 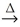 2 Pb(l) + CO2(g)
2 Pb(l) + CO2(g)
How much ore that is 5.2% PbS is necessary to produce 12.0 kg of Pb?
A) 0.721 kg
B) 200 kg
C) 2.67 kg
D) 133 kg
E) 267 kg
Correct Answer:
Verified
Q101: Complete the reaction:
SiO2 + 2 C
Q102: What average current must be maintained for
Q103: How much tin is needed to make
Q104: What weight of lead sulfate is produced
Q105: Bronze is 90% Cu and 10% Sn
Q107: The molecular geometry for HCN is _.
A)trigonal
Q108: Complete the following reaction:
B(OH)3(aq)+ 2 H2O(l)→
A)B ∙
Q109: How much tin is required to make
Q110: If in a lead storage battery the
Q111: The acidity of the group 3 oxides
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents