The masses of 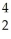 He,
He, 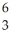 Li and
Li and 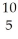 B are 4.0015,6.0135 and 10.0102 amu,respectively.Splitting a boron-10 nucleus to helium-4 and lithium-6 would:
B are 4.0015,6.0135 and 10.0102 amu,respectively.Splitting a boron-10 nucleus to helium-4 and lithium-6 would:
A) absorb energy
B) evolve energy
C) result in no energy change
D) boron cannot be split
E) cannot be determined
Correct Answer:
Verified
Q35: In the case of radioactive element X,which
Q36: Consider the following nuclear reactions. 
Q37: Which of the following would have the
Q38: A wooden artifact is subjected to radiocarbon
Q39: Half-life is:
I.the time for a sample to
Q41: Nuclear binding energy is:
I.the energy liberated when
Q42: Which of the following nuclides is most
Q43: An isotope which has too high a
Q44: Find the INCORRECT nuclear reaction.
A) 
Q45: On forming the nuclide from the protons,neutrons
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents