A hydrocarbon has a molecular weight 56 g/mol and contains 85.7% carbon.It easily adds one mol of HBr to produce a chiral alkyl bromide.Which of the following structures could be the hydrocarbon?
A) 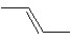
B) 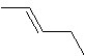
C) 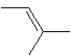
D) 
E) 
Correct Answer:
Verified
Q21: Which of the following compounds could be
Q22: During the nitration of a benzene ring
Q23: What product would you expect to obtain
Q24: Which of the following could be added
Q25: Which of the following groups is a
Q27: What is the major product in the
Q28: Which of the following roles can alcohols
Q29: Which of the following steps would you
Q30: Find a group that is a ortho,para
Q31: The chlorination of benzaldehyde is producing:
A)m-chlorobenzaldehyde
B)a mixture
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents