In the example of ionic bond formation between sodium and chlorine,as shown,which of the following is not a true statement? 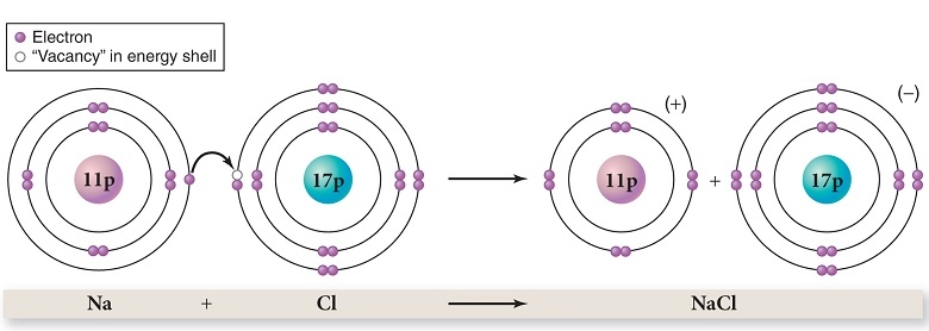
A) Sodium donates an electron.
B) The bond that is formed is stronger than a hydrogen bond.
C) Na is the chemical symbol for sodium.
D) Sodium becomes positively charged.
E) Chlorine donates an electron.
Correct Answer:
Verified
Q26: An element is found to have atoms
Q27: Trees are able to transport water from
Q28: The property of water demonstrated by this
Q29: Carbon and hydrogen make up many biologically
Q30: Algal phytoplankton are single-celled water organisms that
Q32: In a covalent bond,atoms
A) share a proton.
B)
Q33: You can painlessly wade into a pool,but
Q34: A base
A) is a chemical that absorbs
Q35: Organic molecules are defined as chemical compounds
Q36: Evaporation of water is
A) a phase change
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents