The heat of vaporization of water is 540 cal/g. What mass of water at 100.0 °C can be vaporized by the addition of 55.0 kcal of heat?
A) 0.19 g
B) 55 g
C) 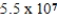 g
g
D) 102 g
Correct Answer:
Verified
Q18: One would expect a compound with the
Q19: Which of the following is true as
Q20: Diethyl ether historically was used as an
Q21: Ethylene dichloride is an effective cooling agent
Q22: When alcohol is mixed with water which
Q24: In which of the following processes does
Q25: Which of the following best describes the
Q26: Glycerol changes its shape to conform to
Q27: Examine the following representation of a change
Q28: Which of the following phase changes does
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents