Copper reacts with nitric acid as given in the following reaction. 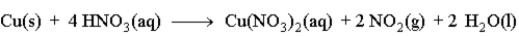 Predict the effect of the given change on the rate of the reaction by filling in the blank with one of the following terms.
Predict the effect of the given change on the rate of the reaction by filling in the blank with one of the following terms.
increase
decrease
no effect
-The concentration of the nitric acid is increased. ______________________
Correct Answer:
Verified
Q43: If a reaction occurs very rapidly, even
Q44: Consider the following reaction. Q45: Which of the following is the best Q46: What is the name of a branch Q47: Consider the following energy diagram showing a Q49: Consider the following energy diagram showing a Q50: Consider a sample of water in a Q51: When the following equation is balanced using Q52: If the 'Nutrition Facts' on a food Q53: Complete the following sentence using the terms![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents