Consider the following reaction. 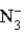 + H2O
+ H2O 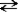 HN3 + OH−
HN3 + OH−
This reaction indicates that:
A) 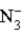 is a weak base.
is a weak base.
B) 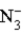 is a strong base.
is a strong base.
C) 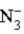 is a weak acid.
is a weak acid.
D) 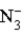 is a strong acid.
is a strong acid.
Correct Answer:
Verified
Q23: Ascorbic acid (Vitamin C), H2C6H6O6, has a
Q24: When a solution of HF (a weak
Q25: An ammonia solution has a pH of
Q27: Which of the following pH's corresponds to
Q28: When fumaric acid, H2C4H2O4 reacts with NaOH,
Q29: A sodium hydroxide solution has a pH
Q30: Which of the following is the conjugate
Q31: Oxalic acid (H2C2O4) is weak acid found
Q36: Which of the following is the conjugate
Q160: Which of the following solutions can function
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents