Which of the following would correctly describe the respective 13C NMR and 1H NMR spectra for the indicated atoms for the compound shown below? 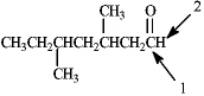
A) Atom 1 would produce a peak at 205 and atom 2 would appear as a singlet.
B) Atom 1 would produce a peak at 195 and atom 2 would appear as a singlet.
C) Atom 1 would produce a peak at 205 and atom 2 would appear as a triplet.
D) Atom 1 would produce a peak at 195 and atom 2 would appear as a triplet.
Correct Answer:
Verified
Q20: Predict the products of the following reactions.
Q21: What is the IUPAC name of the
Q22: Based on the following IR spectrum,
Q23: Draw the product(s)of the following reaction.
Q24: What is the name of the major
Q25: Predict whether the following reactions of the
Q27: What is the correct assignment of the
Q28: Draw the structure of the product obtained
Q29: In the box,what is the name of
Q30: Synthesize the following alkene through the Wittig
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents