Nickel has a lower atomic mass than cobalt, even though it has a higher atomic number.One possible explanation is that one of the average atomic masses was miscalculated.In the case of cobalt, there is only one isotope: 100% 59Co at a mass of 58.9332 amu.For nickel, however, there are five isotopes as given in the table. 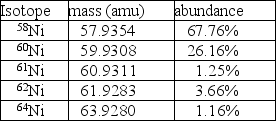 A.Using the data in the table, calculate the average atomic mass for nickel.
A.Using the data in the table, calculate the average atomic mass for nickel.
B.Is the atomic mass for nickel in your periodic table correct?
C.Regardless of your answer to part B, how else could you explain the observation that the atomic mass of nickel is less than the mass of cobalt, even though it has the higher atomic number?
Correct Answer:
Verified
B.yes
C.Cobalt has 27 proton...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q121: Washing soda is a hydrate of sodium
Q125: A 0.8715 g sample of sorbic acid,
Q126: The first step in the Ostwald
Q127: Calculate the molar mass, in g/mol, of
Q128: Aluminum hydroxide reacts with nitric acid to
Q132: Liquid heptane, C7H16 , burns in oxygen
Q132: One way of obtaining pure sodium
Q137: Liquid hexane, C6H14, burns in oxygen gas
Q138: Liquid heptane, C7H16, burns in oxygen gas
Q140: Liquid heptane, C7H16, burns in oxygen gas
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents