A chemistry student determined the empirical formula for tungsten oxide (WxOy).To do so, he heated tungsten with oxygen in a crucible.The data that he recorded are shown below: 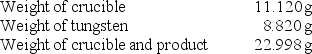 What is the empirical formula of tungsten oxide?
What is the empirical formula of tungsten oxide?
Correct Answer:
Verified
Q144: A chemistry student determined the empirical formula
Q145: What percent by mass of oxygen is
Q146: If 0.66 mole of a substance has
Q147: Calculate the volume of 0.15 mole of
Q148: How many ICl3 molecules are present in
Q150: Calculate the percent composition by mass of
Q151: Phosgene, a poisonous gas used during WWI,
Q152: Calculate the percent composition by mass of
Q153: A sample of unknown ore was analyzed
Q154: A compound with a percent composition by
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents