What is the pressure of the sample of gas trapped in the open-tube mercury manometer shown below if atmospheric pressure is 736 mmHg and h = 9.2 cm? 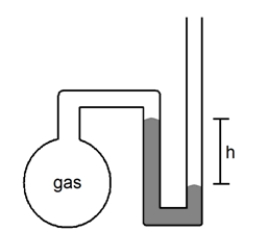
A) 92 mmHg
B) 644 mmHg
C) 736 mmHg
D) 828 mmHg
Correct Answer:
Verified
Q3: Which of the following statements is consistent
Q15: At constant temperature and volume, a sample
Q15: A sample of a gas occupies 1.40
Q17: What will happen to the height (h)of
Q18: What will happen to the height (h)of
Q21: Calculate the density of Br2(g)at 59.0°C and
Q22: Calculate the number of moles of gas
Q23: Gases are sold in large cylinders for
Q23: Calculate the grams of SO2 gas present
Q25: A gas evolved during the fermentation of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents