Determine the pressure of the gas trapped in the apparatus shown below when the atmospheric pressure is 695 mmHg. 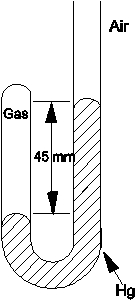
A) 45 mmHg
B) 650 mmHg
C) 695 mmHg
D) 740 mmHg
E) 760 mmHg
Correct Answer:
Verified
Q57: What volume of O2(g)at 810.mmHg pressure
Q58: A 1.17 g sample of an alkane
Q59: How many liters of chlorine gas
Q60: A sample of hydrogen gas was collected
Q63: What mass of KClO3 must be decomposed
Q64: Which statement is false?
A)The average kinetic energies
Q67: Liquid nitrogen has a density of 0.807
Q80: If equal masses of O2(g) and HBr(g)
Q90: A spacecraft is filled with 0.500 atm
Q96: A spacecraft is filled with 0.500 atm
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents